Fertilizers, Calculators
Ready-made fertilizers, like Flora, are, of course, good - but expensive. If you are doing home hydroponics, then it doesn’t bite so much off your budget, but if you have expansions plans for the future... I'm going to retire... I'll buy a farm...
It's cheaper to buy a Bitcoin farm then to fertilize even a mini farm with Flora.
In addition, by tailoring the composition of nutritious solution to your specific needs, you can significantly increase the yield.
So, let's discuss basics of do-it-yourself fertilizers. First of all, you will need to buy the necessary components. They are reasonably cheap, simply because they are used on a blobal scale, so the only way you can buy them at high price is in a small packages in an expensive flowers store. On a farm market they are sold by pounds if not by 20 kg bags. Some components can be found in pharmacies, but again, it will be much more expensive and does not make much sense.
As for dosages, everything has been posted online already. Let's take cucumbers as an example. Do a simple Google search for "hydroponics nutrient solution for cucumbers", and you will get an overwelmimg number of links and about the same number of questions.
What is ppm? 1 mg/l = 1 ppm.
Why do they give the amount of the target element instead of grams of salts that contain it? Because there are many different fertilizers containing the same element (like Nitrogen).
Of course, all this mess was automated long time ago. Go to Google Play (if you use Android) and look for "hydroponics nutrients calculator"; or if you prefer PC, look for an executable for Windows; finally, you can look for "hydroponics nutrients calculator" - and get a program that you can run without any risk of getting viruses.

One more thing: you can also search for such calculators available as Excel tables.
What do we want from such calculator?
We need to be able to set the composition of the solution as the input, and get at the output some recommendations: how much (and of which kind) of fertilizers to weigh. I don't give details because each such calculator, in theory, has a textbook, and it is better to look there. However, there are some important details.
First. As a result of youe efforts, you should receive weight recommendations for the preparation of two solutions: solution A and solution B. This approach is pretty much a standard, so if the calculator you found doesn't do it, you better find another one.
The reason for using two solutions is simple: we want to make concentrated solutions and dilute them whenever we need. The fact is that we cannot mix concentrated solutions - precipitate will form. We need to dilute them first (for example 50:1), then mix.
Second. Some of the solutions recommended in the Internet ignore trace elements, and this is not right. Look for those that mention boron, manganese, iron, copper, and molybdenum.
Or take a ready-made mixture of trace elements, they are sold both in gardening stores, farm markets or in stores for aquarists (for aquarium plants). A good calculator will allow you to enter the content of one of the elements in this mixture (usually, it is boron) and will give the total dosage.
Let's walk through one of the web pages with such a calculator: a typical representative of hydroponic nutrient solution calculators:
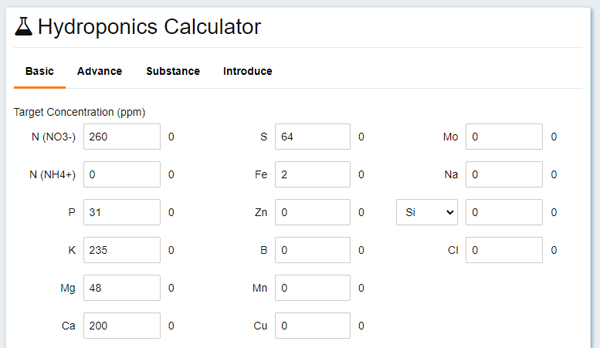
Here you can enter the composition of the solution - the one that you found on the Internet while searching for "hydroponic solution for something".
Concentrations are in ppm, which corresponds to milligrams per liter, that's just these concentrations are for ions: how can we weigh 260 mg of NO3? Well, this is exactly what you need a calculator for - it will recalculate everything for you from abstract "nutritious elements" to something you can weight.
Let's look a bit lower on the page.
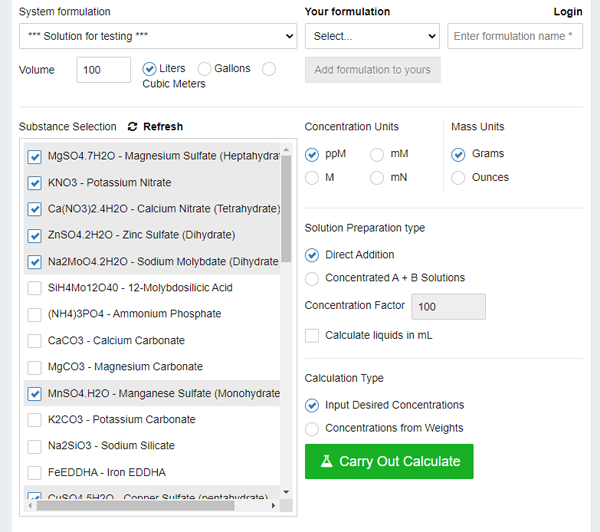
Here you can set the target volume of the solution (by default, 100 liters). One hundred - quite normal for home hydroponics, because as a result we will get it concentrated 20-50 times, that is, two two-liter bottles (which we will then dilute to 50 liters).
Look at the left combo box. Bingo! In the Solution for testing drop-down list, we see ready-made options for different plants: no need to search the web!
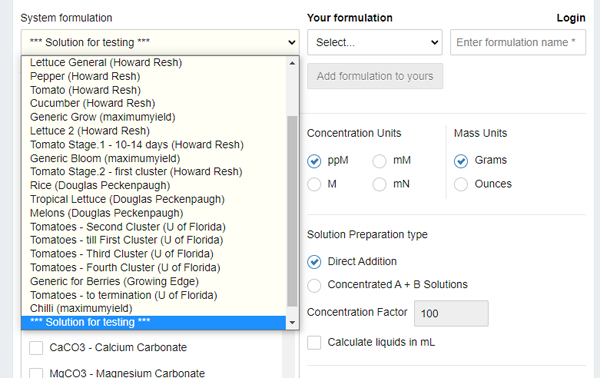
For our example, lets select cucumber.
Of course, here is a pitfall: if you want to add your own composition for a nutrition solution, you will have to register in that particular program. It's not difficult, just what are you going to do, if the site goes down? (use an offline program for Windows, Android or whatever OS you have).
We can also choose units (grams, ppm), as well as two options for the target solution: either we prepare the final solution in a large tank, or, as I mentioned, we make concentrated solutions A and B.
Select A and B, and set the Concentration Factor (dilution ratio) to 50. If you use the one hundred that they have by default, most likelt you will have problems dissolving salts.
In the list at the bottom left, we can check the boxes for salts that we have in stock. The same nitrogen exists in several forms, as well as in combinations with other elements (for example, ammonium phosphate) and we often can not use the package of fertilizer you have, because you cannot calculate dosages. Here the problem is partly resolved.
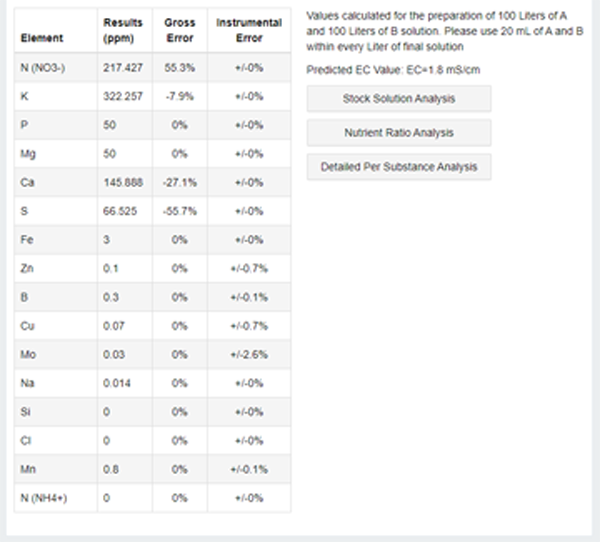
Click on the "Carry Out Calculate" button (it seems that the program was written by the Indian programmers), and get the results:
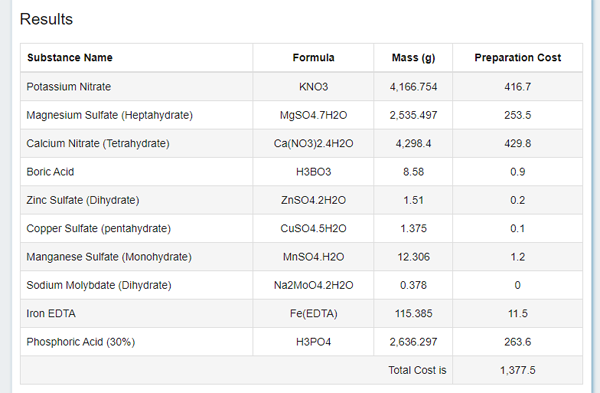
So, we have an option to weigh specific amounts of specific salts, from among those that you can buy at a Agricultural Market. The price is also given there in an unknown (I wasn't able to figure it out) currency, let's just ignore it. What is important: we now have amounts of each component (real components, not a virtual "NO3") we have to weight. And by "real components" I also mean...
... the fact is that salt may exist in few forms that differ by the water content. So if you see in the table the "Whatever Dihydrate", make sure salt you have in stock is also a dihydrate, it should be written on it. Or recalculate the required amounts yourself using school proportions. Or choose a different salt from the list.
As a bonus, they have calculated the target value of the EC (more on that below) for us.
Note that this was just an example. I do not recommend this particular calculator, it is simply the most clear and straightforward of all that I found during the simple search. There are many great calculators awailable, some of them are a little more confusing, however, there also are excellent textbooks and video courses for them.
What do I dislike about this calculator? The idea that the composition of the fertilizer you buy in store equaks to its chemical composition. That is, if we take calcium phosphate, then it will contain as much calcium and phosphorus as we can calculate, knowing the chemical formula and molecular weights of the elements. But it's not always true.
Often fertilizers contain impurities, often "dihydrate" contains a mixture of dididrate and monohydrate and so on. Fertilizer manufacturers know this very well, so usually they write on the package the percentage of the target elements. That is, the fertilizer that contains potassium might have forty percent of it (potassium) according to the formula, and the package says 35. A good calculator will allow you to enter a correction for each salt.
The difference is usually small enough to be neglected, but still...
Fertilizers (concentrated solutions A and B we just made) should be stored in a dark place, in a dark, preferably wrapped in foil, bottles. If you have bacteriostatics (sodium benzoate) - add it to concentrate, because, even in the dark, it can be "eaten" by bacteria. Recommended concentration of sodium benzoate is 100-300 mg per liter (of a concentrate), but I advise you to do your own search on the net, in other words, if you poison yourself - it's your own fault.
Speaking of sodium benzoate. $12 per kilogram, if you buy it at 100-300 grams packages, and 30c per kilogram, if you take a 25 kg drum from the Chinese supplier. These are the paradoxes of hydroponics, they are beyond logic :)